Abstract
Introduction
Immune-mediated thrombotic thrombocytopenic purpura (iTTP), or acquired TTP (aTTP), is a life-threatening thrombotic microangiopathy that requires prompt treatment to improve patient outcomes. Caplacizumab is a von Willebrand factor (VWF)-directed antibody fragment that rapidly inhibits VWF-platelet interaction and prevents microthrombi formation in iTTP. Based on efficacy and safety demonstrated in the Phase 3 HERCULES trial, caplacizumab, in conjunction with therapeutic plasma exchange (TPE) and immunosuppression, is approved in the USA and EU for aTTP. The aim of this Phase 2/3 study (NCT04074187), conducted in Japan, was to evaluate the efficacy and safety of caplacizumab in Japanese patients with iTTP.
Methods
Japanese patients aged ≥18 years with a clinical diagnosis of iTTP who had received ≤1 TPE were enrolled in this single-arm, open-label study. Patients received caplacizumab in conjunction with TPE and immunosuppression, during daily TPE, and for ≥30 days after discontinuation of TPE. Treatment extension was allowed for ≤8 weeks in case of persistent ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) deficiency defined by the investigator and patients were followed for 4 weeks after end of caplacizumab treatment. Primary endpoint was the proportion of patients with a recurrence of iTTP during the overall study period, assessed in the per-protocol (PP) population; recurrence rate ≤20% was the success criterion. Key secondary endpoints were assessed in the PP and modified intention-to-treat (mITT) populations. Treatment-emergent adverse events (TEAEs) were assessed in the safety population. PP population included patients who completed treatment and follow-up per protocol or had a recurrence of iTTP; mITT and safety populations included patients with ≥1 dose of caplacizumab. All analyses were descriptive. The study was conducted in accordance with the Declaration of Helsinki.
Results
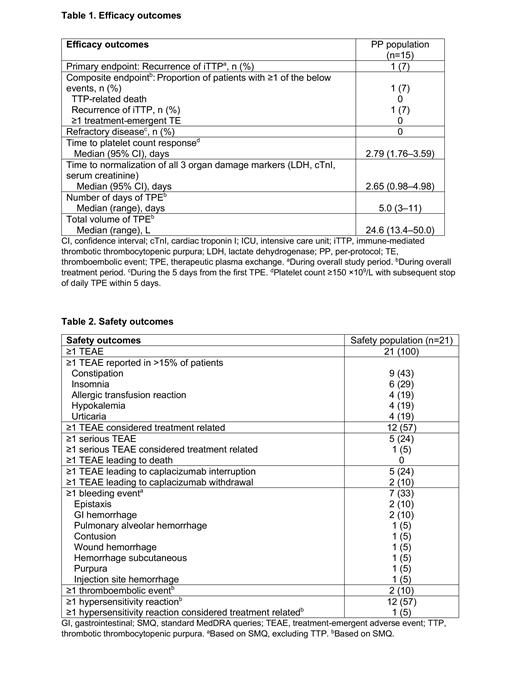
A total of 21 patients were enrolled and treated with caplacizumab; 6 patients discontinued (adverse event: n=2, physician decision: n=4), and 15 patients were included in the PP population. In the mITT population, median age (range) was 59 (22-86) years; 16 (76%) patients presented with an initial episode; median (range) platelet count at baseline was 21.5 (8-78) ×10 9/L;10 (48%) patients received rituximab; median duration (range) of caplacizumab exposure during the overall treatment period was 35 (7-69) days. All patients in the PP population had ADAMTS13 activity <10%. During the overall study period, 1 (7%) patient experienced iTTP recurrence. Median (95% confidence interval [CI]) time to platelet count response was 2.79 (1.76-3.59) days. Additional efficacy endpoints are shown in Table 1. The most common TEAEs were constipation and insomnia, reported in 43% and 29% of patients, respectively (Table 2); 2 treatment-emergent TE events were reported, cerebral infarction and deep vein thrombosis, in 1 patient each. One patient had a treatment-related serious bleeding event of pulmonary alveolar hemorrhage. No deaths occurred during the overall study period (treatment and follow-up).
Conclusions
In Japanese patients with iTTP, caplacizumab in conjunction with TPE and immunosuppression was associated with a low rate of recurrence of iTTP and fast normalization of platelet count and organ damage markers. These findings are comparable to those in the HERCULES study, in which caplacizumab was associated with 12% recurrence rate and median (95% CI) time to platelet count normalization of 2.69 (1.89-2.83) days (Scully M, et al. N Engl J Med. 2019;380 [4]:335-346). Caplacizumab was well tolerated, and no new safety signals were identified in the Japanese population.
Funding
This research was funded by Sanofi.
Miyakawa: Sanofi: Research Funding; Zenyaku Kogyo: Consultancy; Sanofi: Consultancy; argenx: Consultancy, Research Funding. Imada: Celgene Co., Ltd.: Honoraria; Bristol-Myers Squibb K.K.: Honoraria; Astellas Pharma Inc.: Honoraria; Chugai Pharmaceutical Co., Ltd.,: Honoraria; Sumitomo Dainippon Pharma Co., Ltd.: Honoraria; Otsuka Pharmaceutical Co. Ltd.: Honoraria; Takeda Pharmaceutical Co. Ltd.: Honoraria; Novartis Pharma K.K.: Honoraria. Ichikawa: Sanofi: Honoraria; AstraZeneca: Honoraria; Chugai: Honoraria. Handa: Daiichi Sankyo: Research Funding; Janssen: Honoraria; BMS: Honoraria; Ono: Honoraria; Sanofi: Honoraria, Research Funding; Abbvie: Honoraria; MSD: Research Funding; Shionogi: Research Funding; Celgene: Honoraria, Research Funding; Chugai: Research Funding; Kyowa Kirin: Research Funding; Takeda: Honoraria, Research Funding. Matsushita: Shire/Takeda: Honoraria; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bioverativ/Sanofi: Honoraria; Chugai: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Educational and investigational support; CSL Behring: Honoraria; JB: Honoraria; KMB: Honoraria; Nichiyaku: Honoraria; Octapharm: Honoraria; Sysmex: Honoraria; Baltaxa/Shire/Takeda: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Other: Educational and investigational support; Pfizer: Membership on an entity's Board of Directors or advisory committees; Kirin: Honoraria. Hashimoto: Sanofi KK: Current Employment, Other: May hold shares and/or stock options. Ohshima: Sanofi KK: Current Employment, Other: May hold shares and/or stock options. Tahara: Sanofi KK: Current Employment, Other: May hold shares and/or stock options. Tanaka: Sanofi KK: Current Employment, Other: May hold shares and/or stock options. Matsumoto: Sanofi: Consultancy; Takeda: Consultancy; Alexion Pharma: Consultancy; Asahi Kasei Pharma: Research Funding; Chugai Pharmaceutical: Research Funding; Alfesa Pharma: Patents & Royalties: ELISA for measuring ADAMTS13 activity.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal